FDA Approves Shield: A Breakthrough Blood Test for Colon Cancer Detection
The FDA has approved a new blood test called Shield, developed by Guardant Health, Inc., for the early detection of colorectal cancer. This innovative test is designed to identify colorectal cancer-related alterations in cell-free DNA (cfDNA) from a blood sample, offering a less invasive alternative to traditional diagnostic methods.
What is Shield?
Shield is a qualitative in vitro diagnostic test that screens for colorectal cancer in individuals at average risk, aged 45 years or older. By analyzing cfDNA in blood samples collected using the Guardant Blood Collection Kit, Shield detects abnormal signals that may indicate the presence of colorectal cancer or advanced adenomas. Patients with abnormal results are advised to undergo a colonoscopy for further evaluation. It is important to note that Shield is not intended to replace diagnostic or surveillance colonoscopies in high-risk individuals.
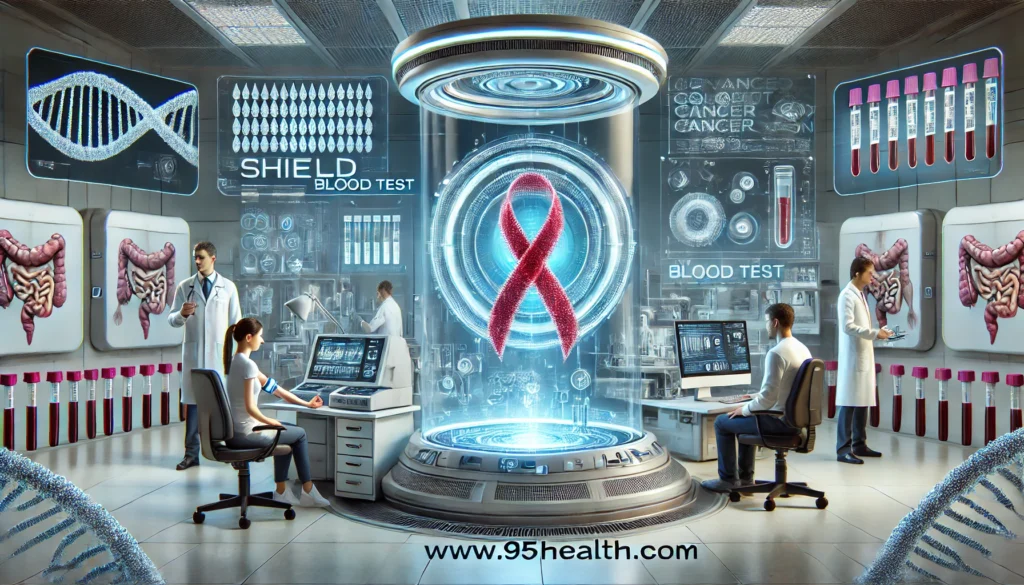
FDA Approval and Benefits
The FDA’s approval of Shield underscores the importance of advancing non-invasive cancer screening methods. The test’s approval followed a thorough review of clinical data demonstrating its efficacy in detecting colorectal cancer markers. The FDA’s Molecular and Clinical Genetics Panel of the Medical Devices Advisory Committee reviewed the premarket approval application and recommended its approval on May 23, 2024.
Shield offers several benefits:
- Non-Invasive: Requires only a blood sample, reducing the need for invasive procedures.
- Early Detection: Facilitates early identification of colorectal cancer and precancerous conditions, which is crucial for effective treatment.
- Convenience: Simplifies the screening process, potentially increasing compliance among patients who might otherwise avoid invasive tests.
Impact on Colorectal Cancer Screening
Colorectal cancer is the third most common cancer in the United States, and early detection is critical for improving survival rates. Traditional screening methods, such as colonoscopy and stool tests, can be uncomfortable and inconvenient, leading to lower participation rates. The introduction of Shield aims to address these barriers by providing a simpler and more accessible screening option.
By leveraging advanced cfDNA analysis, Shield represents a significant step forward in the fight against colorectal cancer. It is expected to enhance screening efforts, particularly among individuals who might be reluctant to undergo more invasive procedures.